Abstract
Background: Systemic AL amyloidosis is caused by the deposition of misfolded monoclonal immunoglobulin serum free light chains (sFLC) in various organs. Treatment of AL amyloidosis relies mainly on chemotherapy aimed at suppressing the underlying secreting plasma cell clone. Organ responses and survival are greatly influenced by the degree of hematological (hem) response evaluated by the decrease in sFLC. Over the last 5 years, anti CD38 monoclonal antibodies (mAb), such as daratumumab (DARA), have emerged as breakthrough targeted therapies for pts with multiple myeloma (MM). CD38, is a transmembrane glycoprotein that functions both as a signal-transducing receptor and a multifunctional ectoenzyme. Its expression is increased in MM and AL amyloidosis plasma cells. DARA received approval in combination with CyBorD in frontline AL amyloidosis1. In the relapse setting, DARA demonstrated a good efficacy and safety profile2,3 but its activity could be enhanced with IMiD®, since it could increase CD38 levels on plasma cells. In AL amyloidosis, various groups demonstrated that pomalidomide (POM) is very effective and better tolerated than lenalidomide, especially in pts with renal insufficiency4,5 with no dose modification (4 mg). Combining an anti CD38 mAb to POM could therefore be an attractive regimen for relapsed pts. Isatuximab (ISA) is another anti CD38 mAb that binds selectively to a unique epitope on the CD38 receptor and has been approved in RRMM in combination with POM or carfilzomib plus dexamethasone (DEX)6,7 with a good safety profile.
Objective: The aim is to evaluate the efficacy and safety of the combination of ISA, POM, and low-dose DEX in pts with AL amyloidosis who did not reach at least very good partial response (VGPR) and/or relapsed.
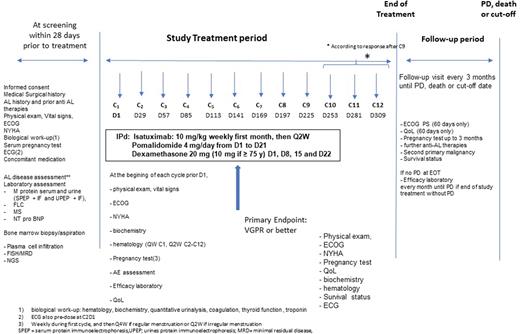
Material & Methods: In this multicenter, single-arm, phase 2 study, we planned to include 46 previously treated pts. Main inclusion and non-inclusion criteria comprise: ≥1 previous line of therapy not in VGPR or better at time of inclusion; measurable hematologic disease: differential of involved and uninvolved sFLC (dFLC)> 50 mg/L; symptomatic organ involvement; adequate bone marrow and organ functions; no dialysis; no overt MM, no cardiac stage IIIb pts; ECOG>2; no previous anti CD38 or POM therapy (if refractory to POM). Pts eligible to enter the study will receive 28-days cycles of ISA (10 mg/kg, IV, weekly for 1st cycle then every-other-week), POM 4 mg (days 1-21) and low-dose DEX (10-20 mg, weekly). The treatment period will be 12 months, unless complete response (CR) at the completion of 9 cycles, disease progression (PD) or unacceptable toxicity occurs. The primary endpoint will be rates of VGPR or better at the completion of 6 cycles to compare with DARA alone in the subset of pts. Secondary endpoints will be: overall hem response rates at various time points; progression-free survival; organ response rates at 1 year; overall survival; time to hem- and organ responses; safety and tolerability; and quality of life (EQ-5D-3L). Exploratory endpoints will comprise: impact of t(11;14) on response; evaluation of minimal residual disease by NGS and by mass spectrometry. This trial is registered as NCT05066607
Results: This phase 2 trial started in February 2022 and, as of August 2nd, 14 patients were screened: 10 are receiving therapy, 1 is in screening and 3 are screen fail. To date, we only observed a transient raised of NT-pro BNP levels due to POM and no unexpected toxicities were reported. DSMB meetings will be called every 6 months or whenever indicated.
Conclusion: This is the first prospective study of ISA in combination with POM and low-dose DEX in AL amyloidosis. Accrual will be updated during the meeting, and patients' characteristics, preliminary results plus safety data will be reported.
References
Kastritis E et al, N Engl J Med. 2021 Jul 1;385(1):46-58
Roussel M et al, Blood. 2020 Apr 30;135(18):1531-1540
Sanchorawala V et al, Blood. 2020 April 30, 135 (18): 1541-1547
Palladini G et al, Blood. 2017 Apr 13;129(15):2120-2123
Sharpley FA et al, Br J Haematol. 2018 Nov;183(4):557-563
Attal M et al; ICARIA-MM study group, Lancet. 2019 Dec 7;394(10214):2096-2107
Moreau P et al, Lancet. 2021 Jun 19;397(10292):2361-2371
Support & Funding: This study received support and fundings from the IFM, the ALLG, BMS and Sanofi drug companies.
Figure 1: study design and flow chart.
Disclosures
Roussel:janssen: Honoraria, Other: travel fees, Research Funding; SANOFI: Research Funding; BMS: Honoraria, Research Funding. Mollee:Amgen, BMS, Caelum, EUSA Siltuximab, Janssen, Pfizer, SkylineDx, Takeda: Membership on an entity's Board of Directors or advisory committees; Janssen, Pfizer: Research Funding. Sidiqi:sanofi: Research Funding; bms: Research Funding. Horvath:sanofi: Research Funding; bms: Research Funding. Karlin:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial Support travel & scientific meetings; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial Support travel & scientific meetings; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene-BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees. Jacquet:Janssen: Honoraria, Other: travel fees. Bridoux:SANOFI: Research Funding, Speakers Bureau; ASTRA ZENECA: Consultancy, Speakers Bureau; Janssen: Consultancy, Honoraria, Other: travel fees, Speakers Bureau. Jaccard:sanofi: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria; Amgen: Honoraria. Gibbs:Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; BMS: Consultancy; Pfizer: Consultancy, Honoraria.
OffLabel Disclosure:
Isatuximab and pomalidomide in relapsed AL amyloidosis
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal